Take a look around: what can be made from oil
Many of the objects around us are more or less oil. Clothes, a toothbrush, a TV, an electric kettle, a lamp, dishes, toys, and many other items that we use in everyday life are made of plastic, and, therefore, are the result of the chemical industry with the use of oil.
Oil is one of the most valuable and widely used raw materials. The states that own its vast deposits, one might say, control the world economy and processes.
For thousands of years, people have studied natural resources and tried to extract useful qualities from them. Having studied the structure of oil, chemists have found that many useful products can be made from it, and now a person's life is surrounded by many objects, things and means that are made of black gold. Under a certain pressure and temperature, various unnecessary impurities are removed from the oil and pure oil products are created.
Oil objects that surround us:
- Fuel;
- Plastic;
- Polyethylene and plastic;
- Synthetics;
- Cosmetical tools;
- Medicines;
- Household and household items.
It is almost impossible to list all products that are based on petroleum. The total number can be determined by a figure within 6000 of such products.
How to make an economist yourself?
But are there any options for making such a device with your own hands at home? Of course, there are ways.

In order to make your own fuel economy, it is enough to take two semicircular magnets based on neodymium. Then we attach these magnets with tape or electrical tape to the fuel hose. That's it, the construction is ready.
As you can see, there are absolutely no problems in making an economist with your own hands. It is done quite simply and without wasting time.
Its advantage over the purchased FuelFree saver is that you absolutely do not spend your money. Even if the fears are confirmed and the effect from the economy is zero, you will not lose anything.
The only open question that requires clarification is where to get neodymium magnets? They are available in sufficient quantities in various electronics. For example, these magnets can be removed from an unusable hard drive on a computer.
You can also try to use a broken magnet from a regular speaker, but in this case you need to be prepared that the effectiveness of the device will significantly decrease. By the way, in many fakes of the original FuelFree, an ordinary magnet was just used, which, to some extent, was the reason for a large number of negative reviews about the device.
What is made from coal: making gasoline at home
Experts say that in order to make gasoline from coal just at home, there are two very interesting and proven methods. They were developed by German scientists in the early years of the last century. During the Great Patriotic War, all German equipment was powered by coal diesel fuel. After all, there were no oil fields in Germany and the Federal Republic of Germany, but the extraction and processing of coal worked smoothly. The Germans made liquid diesel fuel and excellent synthetic gasoline from brown coal.
In terms of chemical compounds, coal is not very different from oil.They have one base - hydrogen and the combustible element carbon. True, there is less hydrogen in coal, however, a combustible mixture can be obtained if the hydrogen indicators are equalized.
One ton of coal can produce up to 80 kg of gasoline. However, at the same time, our coal should contain about 35% of volatile substances. At the beginning of processing, coal is crushed to a pulverized state. After that, the coal dust is well dried, mixed with fuel oil or oil to obtain a pasty mass. After adding the missing hydrogen, the raw material is placed in a specialized autoclave and heated to a temperature of 500 degrees, while pumping a pressure of 200 bar.
Making fuel for cars at home. Is it possible?


A little about the technology for creating ethanol (ethyl alcohol) and biodiesel fuel at home. INTRODUCTORY ARTICLE. NOT A GUIDE TO ACTION!
Problem: Can I make fuel for my car at home?
Watching modern reality shows, I involuntarily wondered, is it possible in reality to make fuel for your car on your own at home? I understand that it is unrealistic to make natural gasoline under artisanal conditions, but is it possible to get some derivatives from it or another type of fuel? Cars drive on wood or water. What kind of car fuel can you make yourself?
Answer:
If you are looking for an alternative fuel or you are spending your time thinking about various apocalyptic scenarios, eat only two really working options that are compatible with the engine systems that we install on our cars and trucks, ethanol (one of the most suitable substitutes for gasoline) and biodiesel (respectively replaces diesel fuel). Both options can be used to replace industrial fuels. Moreover, biodiesel can be poured into the tank of a conventional diesel engine practically without any major changes. Ethyl alcohol is mixed in certain proportions with gasoline, from 10 to 85%. Attention! Not all gasoline internal combustion engines are capable of working on such a mixture.
However, it is not so easy to make the two above-mentioned substitutes for standard fuels. Before trying to make ethanol and biodiesel at home, you will need to study professional literature, purchase (or build) equipment, create a functioning system capable of producing the required amount of fuel of the required quality. Of course, do not forget about safety and do not need to be pushed around by studying the legislation of the country in which you are located. It is possible that the production of certain volumes of surrogate fuel may be illegal.
And even if you study all the intricacies of production, it is hardly worth counting on an inexpensive product (unless you have a hectare for sowing for crops from which you can extract alcohol), the ingredients of a high-octane potion will also cost a pretty penny and the more expensive it will be, the smaller the wholesale you order.
Despite all the difficulties with the study of a new production technology, the purchase of expensive raw materials, the fuel creation technology itself will be simple.
Gasoline from garbage at home: expert opinion
After performing some research, the scientists of the Tomsk Research Institute came to the conclusion that gasoline can be made from a lot of waste that we throw in the trash, without even thinking about its possible further use.
Experiments of scientists have proved that from one kilogram of crushed plastic ordinary bottles, about one liter of fuel is obtained - gasoline.
These scientists in Tomsk have developed a special unit that converts carbon-containing waste into synthetic fuel.Its action lies in the fact that under the influence of high temperature in plastic, carbon-containing substances are destroyed, and as a result of the synthesis of hydrogen and carbon, the necessary gasoline molecules are obtained. And when a large amount of gasoline is produced, you can get fuel oil, gasoline of any brand, and diesel fuel.
Scientists say that today you can get gasoline yourself not only from plastic bottles, for this are suitable:
- Rubber tires;
- Rubbish;
- Firewood;
- Pallets;
- Leaves;
- Shells of nuts;
- Husk from seeds;
- Waste sawdust and rubber;
- Corn rods;
- Peat;
- Straw;
- Reed;
- Weeds;
- Cane;
- Old sleepers;
- Dry manure of birds and animals;
- Medical waste.
And this is not a complete list of items that are suitable in order to extract from them the substances that are so necessary to ensure vital activity.
Making ethanol at home
The process of making ethanol at home is very similar to home brewing.
From which the very first problem immediately follows - the legality of this act. You will need to find out the maximum volume of goods produced and the regulation of alcoholic products in our (in your) country.
Regardless of the amount of alcohol that you produce, you will also have to go through the process of denaturing it, making it unfit for human consumption by adding certain substances to it, such as kerosene or naphtha.
Another important difference between the distillation of moonshine and the fuel itself is that this same ethanol intended for use as a fuel must be more thoroughly refined than the same ethanol intended for human consumption. It should contain less water. Reducing the water content can only be achieved through several distillation steps. There are also some that are capable of removing water contained in fuel alcohol.
When using this ethanol, it would be nice to put additional cleaning filters on the car itself in order to separate water and other debris specifically from the fuel, since ethanol itself, acting as a solvent, will simply wash off all this dirt from the fuel lines and carry them straight into the cylinders.
The process of making fuel is similar to making alcohol. It starts with the selection of raw materials. The initial product can be anything from the same corn and wheat to millet or Jerusalem artichoke.
The raw material is used to make the mash;
Then the fermentation process begins, which breaks down the starch into sugars;
The alcohol is ready.
Getting gasoline from rubber tires with your own hands
Oil is a flammable liquid that is of natural origin, It consists of all kinds of hydrocarbons, as well as a certain amount of other organic substances. The production of gasoline from oil extracted in the ground is the lot of oil refineries, however, as an interesting experiment, it is possible to obtain it in small quantities at home.
To do this, you will need:
- 3 refractory containers;
- Rubber waste;
- Distiller;
- Bake.
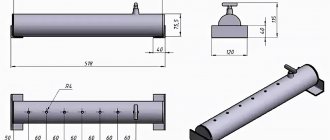
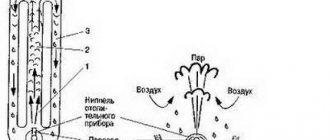
Move children away. Having prepared a container with a tight-fitting lid, you must attach a heat-resistant tube. This will be our retort. For a condenser, any container is suitable for us, and in order to make a water seal, it is necessary to find a strong vessel with two tubes. It is necessary to assemble this device for liquid hydrocarbons, connect the pipe from the retort lid to the condenser, and insert the hose. Connect its second end to the water seal tube. We connect the second shutter tube to the furnace and put a retort on it. We get a closed system for the production of high-temperature pyrolysis.We just have to load rubber tires and wait for the gasoline at the exit.
Getting 92 gasoline
Our article What is gasoline tells in detail about what brands of gasoline are and how they differ. Let's talk more about how to make 92 gasoline (high octane). To do this, you need to heat the mixture to the highest possible temperature. At the proposed 200 ° C, the gasoline yield will be only 10% or less, and if the column is heated to 500 ° C, the yield will be much greater. The resulting combustible mixture can be brought to 95th gasoline using octane-increasing additives.
The gasoline obtained in the described way is practically no different from that which is sold at gas stations. True, the octane number of such a fuel can be lower, therefore it is undesirable to use it in expensive cars, as it can damage the engine. In addition, homemade gasoline needs additional purification from impurities.
How to make gasoline at home (video)
Oil is by far the main source of energy and synthetic materials on Earth. It's hard to imagine our world without cars, electricity, airplanes and more. A lot depends on oil, and it seems that we ourselves are dependent. But isn't it time for us to find other, alternative ways of extracting fuel from the funds that lie under our feet? It's so easy to take and recycle the garbage. Much easier than depleting natural resources and depending on those who extract them.
Gasoline has become scarce - many motorists are thinking about what else to invent to save it, or even replace it. Ideas come up, disputes arise. It turns out, however, that not all of their participants have a clear idea of what the current motor gasoline is. It is to this topic that we decided to devote our today's lecture, prepared on the basis of literary sources.
Gasoline is known to be derived from petroleum
... This natural liquid basically consists of only two chemical elements - carbon (84-87%) and hydrogen (12-14%). But they combine with each other in a great variety of combinations, forming substances that we call hydrocarbons. A mixture of various liquid hydrocarbons is oil.
If oil is heated at atmospheric pressure, then the lightest hydrocarbons evaporate from it first, and as the temperature rises, the heavier ones. Condensing them separately, we get different fractions; those of them that boiled away in the temperature range from 35 ° to 205 ° С are considered gasoline (for comparison, condensate obtained at temperatures from 150 to 315 ° С is called kerosene, from 150 to 360 ° С - diesel fuel).
However, this method (called direct distillation) produces very little gasoline - only 10-15% of the distilled oil. A huge fleet of cars that need this type of fuel cannot be “fed”. Therefore, the bulk of commercial gasoline is produced as a result of the so-called secondary oil refining processes, which include thermal and catalytic cracking, platforming, reforming, hydroforming and many more. These processes are complex, but they are united by a common goal - to break up large and complex molecules of heavy hydrocarbons into smaller and lighter ones, which form gasoline. Without going into the technological details of secondary processing, we only note that it allows not only to increase the yield of gasoline from oil several times, but also provides a higher quality product compared to direct distillation.
So, light petroleum fractions, which can serve as fuel for carburetor automobile engines, have been obtained and from them it is necessary to prepare commercial gasoline with certain properties. We will talk about these properties.
Heat of combustion. The chemical energy contained in any fuel, when burned, is released in the form of heat, and it can be turned into mechanical work.This is exactly what happens in the motors of our cars. The specific heat of combustion of motor gasolines is a fairly constant value, each
a kilogram of this fuel emits about 10,600 kilocalories - a serious charge of energy, which is sufficient, for example, to lift a weight of 4,500 tons to a meter height.
Octane number
... In a mixture of gasoline vapors with air, which is compressed in the combustion chamber of the engine, the flame spreads at a speed of 1500-2500 m / s. If the compression is too great, peroxides are formed in the combustible mixture, and combustion becomes explosive. This is the detonation well known to motorists, which leads to an emergency engine failure.
The knock resistance of gasoline is assessed by its octane rating. It is determined by comparing the test gasoline with a special reference fuel consisting of a mixture of isooctane (its octane number is taken as 100) and heptane (taken as zero). How many percent of isooctane is in the mixture on which the engine operates in the same way as on a given gasoline, such is the octane number of this gasoline.
Of course, the motor setup in this experiment is special, research, and all the conditions of the experiment are standardized. If we talk about driving under normal operating conditions, then it would be wrong to attribute detonation only to the properties of gasoline itself. The danger of its occurrence increases due to the following: large opening of the throttle valve in the carburetor, lean fuel mixture, increased ignition timing, increase in engine temperature, decrease in crankshaft speed, large amount of carbon deposits in the cylinders, unfavorable atmospheric conditions (high temperature and low air humidity, high barometric pressure). By the way, the combination of these very factors often leads the driver to erroneous conclusions, they say, bad gasoline was poured at the gas station, or vice versa - this is what a good engine, even on low-octane gasoline, does not detonate.
It should be noted here that the octane number of gasoline is determined primarily by which fractions, which hydrocarbons prevail in it. High-octane components include alkyl gasoline (a mixture of aromatic hydrocarbons), toluene, isooctane, alkylate (a mixture of isoparaffinic hydrocarbons).
However, it is possible to increase the octane number of gasoline by adding a special additive to it - an antiknock agent. Until recently, tetraethyl lead (TPP) or tetramethyl lead was widely used for this purpose, preparing leaded gasolines known to all. But when they are used, lead oxide is deposited on candles, valves and walls of the combustion chamber, and this is harmful to the engine. The main thing, however, in another thermal power plant is a strong poison, its presence in the exhaust gases poisons the atmosphere and harms people and all living things in general. Therefore, now everywhere, including in our country, they refuse to use ethyl liquid, despite the associated increase in the cost of gasoline.
The fractional composition objectively characterizes the volatility of motor fuel. The lower the temperature at which 10% of gasoline is distilled, the better its starting properties, but the greater the risk of vapor plugs in the fuel supply line, as well as icing of the carburetor. The relatively low distillation temperature of 50% gasoline testifies to its good volatility in operating modes, but again to its ability to cause icing. Finally, a high distillation temperature of 90% indicates that there are many heavy fractions in gasoline, which contribute to the dilution of the oil in the crankcase and the associated deterioration of the lubrication of engine parts.
We just mentioned steam plugs and carburetor icing. The first, obviously, does not require special explanations, since this phenomenon is familiar to every car enthusiast.It should only be noted that for commercial gasolines supplied to filling stations during the cold season (from October to March inclusive), the distillation temperature of 10% of the total volume is 55 ° С, and in summer - 70 ° С. That is why "winter" gasoline, stored until hot, while driving can be pretty tormented by steam jams, especially in traffic jams.
As for the icing of the carburetor, a few words should be said about it. Evaporation of a liquid is always associated with the absorption of heat and cooling of the evaporation zone. It's the same with the carburetor. One of the real experiments showed that at an air temperature of + 7 ° C, two minutes after starting the engine, the throttle valve cooled down to -14 ° C; if there are no protective measures, the formation of ice in such a case is inevitable. The main of these measures is the intake of air into the air filter from the exhaust pipe zone ("winter" position of the intake). It should be borne in mind that the conditions in which icing of the carburetor is a real danger are as follows: air temperature from –2 ° to + 10 ° С, relative humidity - 70–100%. The conclusion is simple: although many carburetors are liquid-heated, and a special anti-icing additive is introduced into modern commercial gasolines, nevertheless, with the arrival of cold weather, one must not miss the moment and promptly switch the air intake to the winter position.
Resin formation
... Over time, chemical reactions can occur in liquid hydrocarbons, resulting in the formation of sticky, rubbery substances called resins. They are very harmful as they clog the carburetor and deposit on the intake valve stems. The predisposition of one or another commercial gasoline to gum formation can be different, it depends on the fractional and chemical composition of the mixture, but there are general external conditions that should be borne in mind. Let's list them. The more gasoline comes into contact with the air, the faster resin is formed in it, so resinification in a car tank is much faster than in a filled and sealed canister. Heat and light, as well as the presence of water, accelerate the precipitation of the tar. The material from which the container is made also plays a role: copper and lead enhance gum formation.
Hygroscopicity
... In principle, water does not mix with pure gasoline; it sinks to the bottom of the vessel and remains there as a separate layer. But a very small amount of it (60-100 grams per ton of gasoline) still goes into solution. In aromatic hydrocarbons (benzene, toluene), the solubility of water is 8-10 times higher, therefore, in those commercial gasolines, where there are such components, although a small, but still noticeable amount of water can be contained. This is not a hindrance for fuel combustion, however, if the solution is saturated, then under certain conditions (say, when the temperature drops), water can be released from the fuel and cause considerable trouble - form ice crystals in the carburetor metering elements or promote their oxidation. Therefore, gasoline should be protected from water as much as possible.
Of course, today we have mentioned far from everything that concerns gasoline and is of known practical interest for motorists. “Behind the scenes,” we still have topics that deserve a separate discussion: about the assessment, labeling, features and assortment of commercial gasolines. But a few words about the composition of the two most common brands today must be said here.
Gasoline A-76
... It is based on the product of catalytic reforming or catalytic cracking, which is mixed with gasoline from thermal cracking or straight distillation. To obtain the desired octane number, either ethyl liquid or high-octane hydrocarbon components are added to this mixture.
Gasoline AI-93 in leaded version
is a product of mild catalytic reforming (75–80%), to which toluene (10–15%), alkyl benzene (8–10%) and ethyl liquid are added.
Unleaded gasoline AI-93
obtained on the basis of the product of the catalytic reforming of a severe regime (70-75%) with the addition of alkyl benzene (25-28%) and butane-butylene fraction (5-7%).
Along with making homemade biodiesel from vegetable and animal fats, craftsmen at home also get gasoline or a substance similar to it. Chainsaws, motorcycles and even cars use this fuel. True, no one has thoroughly studied the operation of motors on such fuel and the resource capabilities of the units have not been investigated. But the fact is obvious - the motors function as on ordinary gasoline.
There are a lot of technologies for making cheap gasoline with your own hands. The most famous is the pyrolysis method of obtaining gasoline in your garage or workshop.
What does octane mean
Gasoline octane number Is a measure of detonation resistance, or rather an indicator of various types of fuel and their ignition during the operation of the internal combustion engine. With low octane numbers, the use of such fuel is fraught with negative consequences for the engine, due to the detonation of the fuel. The most common: premature wear of valves and seats, as well as burning residues on the walls and surfaces. Therefore, the octane number must be suitable for a particular engine, and we will discuss how to increase the octane number in this article.
How to make gasoline with your own hands?
The greatest yield is obtained when using waste rubber tires, as well as any other rubber products. They need to be crushed by any suitable means to a size that will allow the pieces to be pushed through the feed hole into the reactor - a metal boiler with a hermetically sealed lid with a gas outlet pipe welded into it. A fire is being made under the reactor. The process uses the technology of decomposition of rubber into complex gas components. Rubber sublimes, bypassing the liquid stage, immediately into gas.
The branch pipe is connected to the condenser (refrigerator) through a water seal (so that there is no access to the oxygen reactor). This is the simplest coil placed in cold water or a jacket cooled by running water. In it, the gas is partially condensed into a liquid, which, after additional distillation, will become home-grown gasoline. It is periodically drained through a valve installed at the far end of the refrigerator. That part of the gas that has not condensed is directed further into a tube with holes - the burner. It is ignited using the reactor for additional heating.
The resulting liquid is a kind of oil that needs to be distilled in the second cycle. It is loaded into an apparatus similar to the first, which already operates as a distiller with a liquid heating temperature of no more than 200 ºС. If we divide the liquid obtained as a result of distillation into fractions (according to the order of the distillate portions), then when testing them for the intensity of combustion, you will notice that the former burn like gasoline, the next ones - like diesel fuel or kerosene. A liquid similar to gasoline and is used in gasoline engines.
Hose with pear
Previously, draining the fuel from the tank was carried out in a simple way. The chauffeur took an iron canister, a rubber hose, and some water. The end of the hose was pushed into the fuel filler neck and pulled into the tank to the very bottom. Then the chauffeur took the other end in his mouth and sucked out the gas until it reached the edge of the hose. The main thing at the same time was not to choke on fuel. Then you had to pinch the hose with your fingers, release it into the canister and unclench your fingers so that the liquid flows into the prepared container. The siphon effect began to work, when, according to the principle of communicating vessels, gasoline rose first up from the tank, and then flowed into the tank below the tank.After the overflow, it was important to rinse the mouth with water from the gasoline that got there and not smoke, so as not to get burns to the face.
In general, there are still enough cars on the domestic market with an uncomplicated gas tank design that can survive such an operation.
Meanwhile, on modern foreign cars (and on LADA of the last years of production), a more complex neck with many bends is used. It requires a very hard and thin flexible plastic hose with a length of more than 3 meters. Then he can squeeze through the serpentine highway.
Question answer
The price of a liter of gasoline in different countries of the world. Infographics
Parts stores sell similar hoses, even equipped with a special pear-shaped hand pump for pumping gasoline. It is no longer required to suck it out of the tank.
However, on some models, it is impossible to put the hose into the gas tank, since there is a protection against fuel draining in the form of a metal mesh that prevents the hose from passing. Therefore, it is necessary to drain gasoline in other ways.
Homemade gasoline options
In a similar way, self-made gasoline is obtained from garbage. As the latter, any plastic parts, scraps of polyethylene, polypropylene, PET bottles (ordinary plastic containers), rubber of all grades are used.
Today, handicraft technologies are known for making gasoline with your own hands (it is correct to say - fuel similar to gasoline) from peat, reeds, straw, husks of seeds, corn cobs, leaves, weeds, reeds and other organic and inorganic substances.


Self-made gasoline, few people risk using it for expensive cars, since the technical parameters of this fuel and its effect on fuel equipment are not known. Homemade gasoline remains the result of interesting experiments by competent self-taught techies.
Users have a completely different attitude to biodiesel or other biofuels obtained by industrial technologies that have certificates of compliance with the standards in force in the country.
If you liked our article and we somehow were able to answer your questions, then we will be very grateful for a good review of our site!
In the modern world, gasoline prices are steadily going up, despite the fact that the cost of oil is constantly falling.
In this regard, many are beginning to think about whether it is possible to make gasoline at home and how to do it.
Acetone
Acetone has proven to be a slightly better fuel than solvent. Despite the fact that the rpm does not reach the optimal value, the car rides more confidently than with a solvent. Periodically, you can switch to fourth gear. The motor works practically without detonation and does not smoke. However, the high volatility of acetone leads to the formation of steam locks, so you have to periodically stop and cool the ramp by pouring water on it.
The consumption of acetone is high, not lower than that of the solvent. However, in the absence of better fuel options, a short distance can also be driven on acetone.
Getting from coal
There are two effective and proven methods. Both of these methods were developed by German scientists at the beginning of the last century.
During the Great Patriotic War, almost all German equipment moved with the help of coal fuel.
After all, as you know, there are no oil fields in Germany, but coal mining has been established. The Germans made diesel and gasoline synthetic fuels from brown coal.
Surprisingly, in terms of chemistry, coal is not nearly as different from oil as many believe. They have the same basis - it is hydrogen and combustible carbon compounds. True, there is less hydrogen in coal. A combustible mixture can be obtained by leveling the hydrogen indicators.
This can be done in the following ways:
- hydrogenation or otherwise liquefaction;
- gasification.
What is hydrogenation
Approximately 80 kg of gasoline can be obtained from one ton of coal. In this case, the coal must necessarily contain 35% of volatile substances.
To start processing, coal is finely ground to a pulverized state. Then the coal dust is thoroughly dried. After that, it is mixed with fuel oil or oil to obtain a pasty mass.
Hydrogenation is the addition of missing hydrogen to the coal mixture.
We put the raw materials in a specialized autoclave and heat it up. The temperature in it should be at around 500 degrees, and the pressure should be 200 bar.
In order for gasoline to form, two phases are required:
- liquid phase;
- vapor phase.
Several rather complex chemical reactions take place in the autoclave. Coal is saturated with the necessary hydrogen, and the complex particles that make up it break down into simple ones.
As a result, we get diesel or gasoline. This will depend on the process itself.
Once again, the whole hydrogenation process point by point:
- grinding coal to dust;
- adding oil to it;
- heating in an autoclave at high temperature.
It is very important to make the right equipment. It is quite difficult to make it yourself at home, because the pressure in autoclaves is higher than in oxygen cylinders.
It is important:
remember safety precautions. The process itself is quite explosive. Never smoke near the unit or make a fire.
Gasification
Gasification is the decomposition of solid fuels into gases.
Later, the missing substances are added to the resulting gases and transformed into a liquid state to obtain gasoline.
There are several ways to convert coal into gasoline using the gasification method.
The first method can theoretically be used at home. It is called the Fischer-Tropsch method. But this method is quite laborious in execution, requires too complicated equipment, and in the end it turns out to be unprofitable, since a lot of coal is spent and the finished gasoline is cheaper.
In addition, a large amount of carbon dioxide is released, the processing process becomes very dangerous at home. Therefore, we will not analyze this method in more detail.
There is also a thermal gasification method. It is carried out by heating raw materials in the complete absence of oxygen. Naturally, this also requires the appropriate equipment. After all, the temperature of decomposition of coal into gas is 1200 degrees.
The main advantage of this method is that part of the gases is sent for the synthesis of gasoline fuel, and part for heating the raw material. This helps keep costs down. Thus, the coal heats up itself.
Water as fuel


All the years of the war, Nazi Germany suffered from a lack of fuel. There are no own oil reserves on its territory, all that was left was to rely on Ploiesti, the field of pro-Hitler Romania, and hope for the Fuhrer, who promised to conquer the oil-rich Transcaucasia for the Fatherland.
But it was not won, and therefore every liter of gasoline was saved. Chemists partially saved the situation: they managed to create synthetic gasoline from coal, but still this gasoline was inferior to natural gasoline. The blitzkrieg failed, the war took on a protracted nature, in which fuel truly became living water.
Imagine the surprise of Gerd Hegel, the head of the concentration camp near the mining village of Kleinwalde, when he was told that one of the prisoners claims that he is able to solve the fuel problem. He summoned the prisoner to him. Unlike Auschwitz or Dachau, the Kleinwalde camp specialized not in the extermination of people, but in the extraction of coal. Russian prisoners of war worked in it, and since it was not necessary to count on new receipts of prisoners in the summer of 1944, it remained to take care of what was.It is impossible to fulfill the coal plan without working hands, and for failure to fulfill the plan, at best, they were sent to the Eastern Front. Therefore, the prisoners were treated relatively gently, avoiding pointless waste of human resources. The last batch of prisoners arrived from near Lviv a few days ago, from which there was a person who asked for an audience.
Hegel accepted it partly out of curiosity, but there was hope in his soul: suddenly the Russian was a talented mining engineer, chemist or inventor. In the Russian army, rank-and-file intellectuals, sometimes scientists, and sometimes doctors of sciences, often served. It would be wasteful to send them to unskilled jobs without first figuring out whether their knowledge of great Germany could be useful.
The presentiment did not deceive Hegel: the prisoner possessed exactly what the Reich needed - the secret of cheap, practically free fuel. This fuel was ordinary water!
Attempts to use water as a source of energy have been made for a long time. You can decompose water into hydrogen and oxygen by electrolysis, and then use hydrogen as a fuel and oxygen as an oxidizing agent. The catch is that the decomposition of water into its constituent parts requires more energy than the reunification of hydrogen and oxygen: the efficiency of any system is less than one hundred percent. Controlled thermonuclear reaction in the early forties existed only in the minds of theoretical physicists. And finally, water is used as a working fluid in hydroelectric power plants. But the prisoner suggested something completely different: to utilize the power of intermolecular bonds of water. It is known that water molecules are packed perfectly: water is incompressible. But if you can weaken the interaction of molecules, make the water loose, then energy will be released, and a lot of energy. Water can be compared to a tightly compressed, locked, powerful spring. If you open the lock, the spring will straighten and do useful work. It is estimated that a liter of water will release as much heat as is generated by burning one and a half liters of gasoline.
But how to do this in practice, how to release the spring, asked the interested Hegel. Simply, the prisoner replied. He watched the thermal waters and came to the conclusion: they sometimes heat up to very high temperatures, because in the depths they come into contact with a certain substance that plays the role of a catalyst. This catalyst also loosens the intermolecular bonds of water. He spent seven years in Kamchatka, in the valley of geysers, trying in various ways to get catalyst from boiling water fountains. And in the end it succeeded! He can demonstrate the catalyst right here and now!
The prisoner took out from the folds of his clothes a nondescript, in appearance iron, mesh plate the size of a rose petal. This is the catalyst. It is worth placing it in a locomotive boiler - and the locomotive will be able to move without coal, feeding on water alone. As befits a catalyst, it itself is not consumed during the reaction, but metal salts are capable of "poisoning" it, so the water must be clean. The only thing that will be required is to timely change the waste water (it will become lighter in the process of breaking molecular bonds and increase in volume, a liter of it will weigh about six hundred grams) into ordinary, fresh water. There were no extra steam locomotives in Kleinwald, and they did it easier - they threw a plate into a bucket of water. The water heated up to a boil in a few minutes - and boiled until it turned into steam. The plate, as promised by the prisoner, remained unharmed.
After conducting the experiment several times (all of a sudden, this is just a trick), Hegel made sure that the prisoner was not lying. He transferred him from underground work to the office, and he himself contacted the relevant departments.
The commission arrived - well-known, authoritative scientists. The prisoner demonstrated with them his method of obtaining energy from ordinary water.By this time, Hegel had prepared an old steam engine, and it, without any fuel, connected to a generator, generated electricity from the well water! It was an energy revolution!
The only drawback of the method was the need for a rare earth catalyst, but the prisoner believed that it was possible to find it in an amount that would meet all the necessary needs in the area of active volcanoes or geysers, by landing troops on Kamchatka or Iceland. If military action is difficult for strategic reasons, an expedition should be sent to Antarctica, to the Erebus volcano. The proximity of the pole guarantees a particularly rich catalyst content. And so they did! In the late autumn of 1944, three ships sailed to the shores of the ice continent. In April 1945, one ship returned to Germany, but it delivered several kilograms of the mysterious catalyst. However, the fate of the Third Reich was a foregone conclusion, there was no time left to remake tanks and armored vehicles for new fuel.
They tried to use the catalyst as a weapon at the very end of the war. When Berlin was practically taken by Soviet troops, the German command flooded the subway with an insidious calculation: a catalyst placed in dozens of places would turn the Berlin subway into a bubbling steam boiler that would explode itself and blow up the fallen capital. But this did not happen. According to some reports, the catalyst deployment group was intercepted by a Soviet special task force. According to others, the Nazi responsible for the operation replaced the catalyst with ordinary iron. The war was lost, and he decided to get to the Americans, and not get through empty-handed ...
Making gasoline from old tires
You can make gasoline with your own hands using old rubber tires.
This will require:
- rubber waste;
- bake;
- distiller;
- containers made of refractory materials.
Expert advice:
it is not worth making gasoline in a city apartment. The process is accompanied by smoke with a pungent odor of rubber.
Step-by-step instructions for making gasoline from rubber tires are as follows:
- It is necessary to prepare a metal barrel with a tight-fitting lid. In addition, a heat-resistant tube is required. It must be connected from above to the cover. Thus, you will get a homemade retort. Then you need a container for condensate and another small container with two tubes to create a water seal. One tube is lowered into the water, and the other is held over it.
- Next, you need to assemble a device for the production of carbon in liquid form. To do this, we connect a tube from our retort to the condensate. Then we also connect the condensate and the water seal with a hose. We connect the second tube to the stove, on which we install the retorts. The result is a closed-loop system for cracking at high temperatures.
- We put the rubber in the retorts and close it tightly with a lid, then it is necessary to heat it over high heat. At high temperatures, rubber molecules are destroyed. Sublimation occurs, that is, a transition from a solid state to a gaseous state bypassing the liquid stage. This gas then enters our condenser, where the temperature is much lower. Vapors condense, and as a result, we get oil in liquid form.
- The resulting substance must be purified; this requires a distiller, which is often used when using moonshine stills. The suspension is brought to a boil at a temperature of 200 degrees, and gasoline is obtained.
Note:
avoid open flames during the distillation process. It is best to use an electric stove.
Propane-butane
Widely used in everyday life for cooking and heating premises, propane-butane proved to be suitable as a surrogate fuel for a gasoline engine.The gas was supplied from the cylinder through a tube connected instead of the crankcase ventilation hose. Fortunately, the diameter of the tube and the hole for the hose almost perfectly matched. The engine started on the first try. After placing the gas cylinder in the salon, we managed to get under way and drive the control segment. True, the engine power dropped significantly, and even on a flat area, it was not possible to engage the fourth gear.
It was possible to place a Korean tin can with butane gas directly under the hood, but this limited its usability in comparison with a propane-butane "summer cottage" bottle. The burner, through which the gas enters the engine, is cooled so much that it becomes covered with frost, so that every two hundred meters it is necessary to stop and warm it up. In addition, the low capacity of the cylinder is unlikely to allow one to travel more than two kilometers on one.
The results of the experiment allow us to safely state that a gasoline engine can work, using not only gasoline as fuel, but also almost any flammable liquid. True, such fuels as rocket heptyl, liquid hydrogen and nitroglycerin have not been tested as fuel samples, but these dangerous and toxic substances are unlikely to be found in the trunk of an average motorist or in a rural store.
Alternative ways
Gasoline isn't just made from coal and rubber tires.
It can be obtained from garbage, firewood, pellets, leaves, nut shells, seed husks, corn rods, peat, straw, reeds, weeds, reeds, old sleepers, dry bird and animal manure, plastic bottles, medical waste, etc.
The process of making gasoline at home, discussed above, is not as complicated as it seems at first glance. Terms such as hydrogenation, gasification, etc. can be misleading. But in fact, setting up production and making gasoline with your own hands is not as difficult as it seems.
We bring to your attention an interesting report on how to make gasoline at home:
If we consider the question of what gasoline is made of, then, of course, many can immediately say that it is from oil. This is true, but this is just the tip of the iceberg, and the actual process of fuel production is much more complicated.
Watch the video
How to make gasoline?


Gasoline is a hydrocarbon-containing substance that is obtained from the distillation of petroleum in industry. It is most often used in internal combustion engines to convert chemical energy into mechanical energy. In our article What is gasoline, this concept is disclosed in detail.
Since gasoline and other automobile fuels are becoming more expensive every day, many are thinking about how to make gasoline from waste, which will be much cheaper than industrially produced one.
Gasoline at refineries
So, it's worth saying right away that the production process is a long process that requires patience and knowledge of chemistry.
32 Gasoline is produced in Russia. This number of industrial capacities allows the Russian Federation to maintain a high grade of fuel. What is gasoline made of? Crude oil is of course the starting material for producing this. Take oil as an example. To make it clearer, 1 barrel is 159 liters. It is also important to note that when crude oil is refined, its volume is constantly increasing and reaches 168 liters. As a result, the following amount of fuel can be obtained from this volume:
- 102 liters of regular gasoline.
- 30 liters of diesel fuel.
- 25 liters of fuel used by aviation.
- 11 liters of refinery gas, which is obtained by the distillation of oil.
- 10 liters of secondary product - petroleum coke.
Obtaining raw materials for the production of flammable alcohol at home


The biggest problem with creating flammable alcohol at home now, or in some hypothetical, apocalyptic future, is raw materials. In order to make a mash that can be distilled into fuel alcohol, you need some kind of seed or other plant material in large quantities. If you have to eat where to grow raw materials, there will be much fewer problems in monetary terms.
Ethanol is mainly made from corn. From every 40 acres it is possible to manufacture up to 1500 liters of ethyl alcohol per year... Of other crops, millet showed even greater efficiency, from the same area in 1 year yield exceeded 2200 liters of ethyl alcohol... Under ideal conditions, millet can produce 4500 liters.
In the absence of acreage for the cultivation of corn, millet, sugar beet and other types of cultivated plants, obtaining alcohol at home will not be a viable project.
How gasoline is made
In order to obtain fuel, it is necessary to carry out a number of operations with crude oil. The point is that the initial product consists of a mixture of various hydrocarbons. It is also important to understand that each molecule of this substance contains a different number of carbon atoms. To put it simply, each of these molecules has its own height and weight.
To get gasoline molecules that are the simplest and lightest, it is necessary to heat the crude oil until the more complex and heavier particles break down into simpler ones - gasoline ones. In other words, if we answer the question of how gasoline is made, we can say that it is obtained by thermal treatment of crude oil. However, it is worth adding to this process some more minor processes, such as cleaning and recycling.
Making biodiesel at home
First of all, it is important to initially understand the difference between the same oil and the biodiesel fuel itself.
Vegetable oil (SVO), waste vegetable oil (WVO) and similar animal fats are naturally capable of nourishing, but they are not biodiesel as such.
In the first version, you cannot do without modifications to the engine itself. At a minimum, a coarse and fine filtering system of vegetable oil waste is required. Not a very good option for a motor.
It is preferable to make this biodiesel from SVO or from WVO oils. The process is more complex and involves “breaking down” the chemical structure of fats or oils using methanol and alkali. It is important to take the necessary precautions as both methanol and alkali are toxic.
The process of making biodiesel from SVO, in its most basic outline.
-Heating oil;
-Adding a certain amount of methanol and alkali mixed ingredients, they will facilitate the chemical process known as transesterification;
-The result of this process will be that in the end two products will be released (obtained), namely: biodiesel and glycerin, which will separate and settle to the bottom of this mixture;
-The final stage is drying of methyl esters of fatty acids. Since water itself leads to the development of microorganisms in biodiesel and contributes to the formation of free fatty acids, which subsequently cause corrosion of metal parts.
Store no more than 3 months.
Manufacturing process
If you answer the question of what gasoline is made of with a simple answer - from oil, then this is not entirely true, since there are some impurities in this fuel, but more on that later.
To obtain fuel in its primary form, it is necessary to subject the raw material to primary processing. This treatment is understood as the purification of oil from salts, as well as water impurities. These processes are carried out under the influence of an electric field. The result of this procedure is the separation of water from oil, as well as desalination to the required level.After completing this procedure, they proceed to the thermal treatment of the oil. It is after such procedures that such fuel is obtained - gasoline, gas, diesel.
This is followed by a catalytic reforming procedure. During this very procedure, the resulting gasoline after primary processing is converted into a fuel characterized by a high octane number. However, such as 92nd or 95th, are obtained by mixing different components that have been obtained as a result of different processes of processing crude oil.
Types of environmentally friendly biofuels
The BIO prefix is now often added to labels based on the rules of successful marketing. The issues of preserving the environment and cleanliness are in vogue all over the planet today. Bioproducts, biocosmetics, biological detergents, biological treatment and energy stations, and even dry closets. It came down to the fireplaces and fuel for them.
Structurally, biofuel fireplaces are equipped with a standard burner and a liquid fuel tank. Adjustment of the size of the flame and the rate of combustion of the fuel is carried out by means of a damper.
If you close it completely, then the fire in the bio-focus will simply go out by itself. In general, a biofireplace is a great way to heat a room and add a touch of comfort to it from the reflections of a “fire”.


The bio-fireplace differs from its wood-fired progenitor in the fuel used to obtain the flame - the logs in it are replaced with smokeless fuel in the form of a liquid
Obtaining biofuel for such a fireplace implies the use of renewable natural resources, environmentally friendly technologies and raw materials in production. Plus, burning it should not give harmful emissions into the atmosphere. Humanity cannot do without combustible fuel. But we can make it less harmful.
There are three types of biofuels:
- Biogas.
- Biodiesel.
- Bioethanol.
The first option is a direct analogue of natural gas, only it is not extracted from the bowels of the planet, but is produced from organic waste. The second is made by processing various oils obtained as a result of squeezing oil plants.
As such, the fuel for biofireplaces is the third option - bioethanol. Biogas is mainly used to generate heat and electricity on an industrial scale, while biodiesel is used more for internal combustion engines in automobiles.


When burning, pure ethanol gives a blue, not too beautiful flame, therefore additives are added to the fireplace biofuel to obtain a red-yellow hue
Home fireplaces are most often filled with bioethanol based on denatured alcohol. The latter is made from sugar (cane or beetroot), corn or starch. Ethanol is ethyl alcohol, which is a colorless and flammable liquid.
But most importantly, when burned, it does not emit odors, carbon monoxide and soot. Simply ideal for city apartments, where it is almost impossible to equip a chimney.
Those wishing to make a biofireplace with their own hands will be helped by a step-by-step guide, which we recommend that you familiarize yourself with.
Octane number
If the question of what gasoline is made of has become more or less clear, then very few know what the octane number is. Everyone knows that the name of each brand of gasoline contains an alphabetic as well as a numerical designation. Letters such as A or AI indicate the method for determining the octane number. A - motor process, AI - research. But the numbers that follow, and show the quantitative content of the octane number in the fuel.
Everyone knows that both oil and gasoline are explosive substances. Since gasoline is obtained from oil by refining it, this property does not disappear anywhere. The octane number indicates the knock resistance of the fuel. In other words, the higher it is, the higher the safety of the fuel grade.However, it should be understood that this indicator is relative, and any spark will still cause an explosion.
Octane number and dilution
I still want to talk a little about the dilution of the original gasoline. That is how we get the octane number equal to 92, 95 and 98, which are used now.
The octane number characterizes the resistance of gasoline fuel to detonation, in simple words it can be described as follows - in the fuel mixture (gasoline + air), which is compressed in the combustion chamber, the flame propagates at a speed of 1500 - 2500 m / s. If the pressure indicator during the ignition of the mixture is too high, then additional peroxides begin to form, the force of the explosion increases - this is a simple detonation process, which is in no way useful for the engine pistons.
It is the resistance of the fuel to detonation that is estimated by the octane number. Now there are installations that contain a reference liquid - usually a mixture of isooctane (it has a number equal to "100") and heptane (it has exactly "0").
Then the stand compares two fuels, one obtained from oil (gasoline mixture), the second from isooctane. They are compared if the engines work in the same way, they look at the second mixture and the number of isooctane in it - thus, the octane number is obtained. Of course, this is all ideally laboratory tests.


In practice, knocking can be caused by many other engine malfunctions, such as an incorrect throttle position, a lean mixture, improper ignition, engine overheating, deposits in the fuel system, etc.
To summarize, now alcohols, ethers, alkyls are used as additives to increase the octane number, they are very environmentally friendly, as well as additives for frost resistance... The ratio in the composition is approximately the following - the composition of the Catholic cracking (73 - 75%), alkyls (25 - 30%), butylene fractions (5 - 7%). For comparison, tetraethyl lead was previously used to increase the octane number, it perfectly improves fuel, but it causes severe harm to the environment (all living things), and also settles in the lungs, and can cause cancer. Therefore, now they abandoned it.
Basic properties of gasoline
The main properties of gasoline include such characteristics as its chemical composition, as well as the ability to evaporate, burn, and ignite. In addition, you can also highlight the resistance to detonation and corrosion activity.
It is important to know that all the physical and chemical properties of gasoline fuel will change depending on how much hydrocarbon and what kind of hydrocarbons it contains. For a more illustrative example, you can take the freezing point for gasoline as a basis. In normal processing, the freezing rate of this liquid is -60 degrees Celsius. However, with the use of additional components, this figure can reach -71 degrees Celsius. The vaporization temperature of gasoline is 30 degrees. The higher this indicator rises, the faster the evaporation will occur. It is also important to note that the amount of fuel vapors from 74 grams to 123 grams or more per cubic meter will already form an explosive mixture.
Chemical properties
In order to consider the chemical properties and their stability in gasoline, it is necessary to be based on the most important indicator - the time that these properties remain unchanged. This indicator is the most important, since during long-term storage of fuel, the lightest hydrocarbons begin to evaporate, which greatly reduces the performance of the liquid as a whole. According to the state standards of the Russian Federation, it follows that the chemical composition of any brand of gasoline from 92nd to 98th remained unchanged for five years. This period is prescribed taking into account the storage of explosive fuel in accordance with all the rules.
Mini refinery
Currently, the issue with the production and purchase of fuel is quite acute, since resources are depleted, and because of this, the price of this product is constantly increasing. In light of these events, the question arises as to what is more profitable to buy - gasoline and other fuel - or to produce it yourself. It is important to understand that for most businesses and companies, fuel costs are the most extensive. It is in this situation that many come to consider the idea of a mini-refinery. This option doesn't seem so bad, especially when you consider the cost of fuel and the cost of a mini refinery. Practically every major entrepreneur can purchase such a mini-plant, which can already be said about, say, a region of an entire country.
Getting raw materials for biodiesel production at home


The great thing about biodiesel is that you can make it from a huge range of vegetable oils and animal fats (and you could theoretically even get free raw materials from local restaurants). The process of obtaining raw materials is as simple as one, two, three. Contact local restaurants, find out if they have vegetable oil waste, find a way to transport it home. Done!
In the absence of a ready source of frying oil waste, obtaining the raw materials to create your own biodiesel becomes more difficult. To buy oil in stores to add to the diesel fuel the subject of the invoice.
Another option is to create your own vegetable oil. The process is lengthy and inexpedient. Maybe in the distant hypothetical post-apocalyptic future, when all other resources are exhausted, this will be economically feasible, but not now.
Outcome: With proper knowledge of technology and technical means, it is somewhat easier to make ethyl alcohol for cars than biodiesel. However, without using the grown material for processing, making homemade fuel turns into an expensive pleasure.
Information edition: News of traffic police, road traffic accidents, traffic fines, traffic police, test traffic rules online. Inspection
Refinery types
Currently, you can buy a mini-refinery for oil refining of almost any type on the market. This is the most important criterion, since these industrial facilities have to be operated in a wide variety of climatic conditions. For this reason, the market is saturated with a variety of types of refineries. There are any specimens, ranging from heat-resistant and corrosion-resistant, to "arctic" installations. A wide range of mini-refineries allows you to process the crude product in almost any conditions.
It is worth noting that they themselves can also operate on different fuels. For their operation, you can use natural or liquefied gas, diesel fuel, fuel oil, crude oil. Such a choice of fuel for the operation of the factory itself provides a wide range of possibilities for the operation of the facility, and also allows you to satisfy any individual preferences for the choice of a working fuel product.