One of the many physicochemical processes that have found wide application, both in industry and in everyday life, is electrolysis - the selection on the surfaces of electrodes connected to a current source placed in a solution or melt, their components (pure metal - aluminum, copper, gas, etc.). The main installation inside which this process takes place is an electrolyzer.

Electrolyzer
What is an electrolyser
An electrolyzer is a special installation used to separate its constituents from a solution or melt.
The main characteristics of the electrolyser are:
- The operating voltage for one electrode ranges from 1.8 to 2.0 V;
- Current strength - for the normal course of the electrolysis process, a current is supplied to the electrodes with a value of this characteristic from 5 to 10 A;
- Number of electrodes - the minimum number of electrodes is 2, the maximum is limited by the size of the installation itself and its purpose;
- Dimensions of electrodes - not carbon rods are used as electrodes, but metal plates, the size of which is determined by the purpose of the installation, the current-voltage characteristic of the current supplied to the plates;
- Distance between oppositely charged electrode surfaces - the minimum distance between the electrode plates must be at least 1.5 mm;
- Electrode material - in modern electrolyzers, stainless steel sheet with nickel is used as the material for the anode and cathode.
Also another important characteristic of an electrolysis plant is the use of catalysts.
Such installations are used for the following purposes:
- Obtaining an oxyhydrogen gas, consisting of a mixture of hydrogen and oxygen (Brown's gas);
- Isolation of pure aluminum, magnesium, zinc from their salt melts;
- Purification of water from salts and impurities dissolved in it;
- Application of a thin layer of nickel, zinc that prevents corrosion on the surface of metal parts;
- Disinfection of food products;
- Purification of wastewater from dissolved salts of heavy metals and other harmful substances.
Important! Platinum-electrode made from ordinary iron is used less often in electrolysis installations than from stainless steel, since it oxidizes faster and becomes unusable.
Self-made electrolyzer
Anyone can make an electrolyzer with their own hands. For the assembly process of the most common design, the following materials will be needed:
- stainless steel sheet (the best options are foreign AISI 316L or ours 03X16H15M3);
- bolts М6х150;
- washers and nuts;
- transparent tube - you can use a spirit level, which is used for construction purposes;
- several herringbone fittings with an outer diameter of 8 mm;
- plastic container with a volume of 1.5 liters;
- a small filter filtering tap water, for example, a filter for washing machines;
- non-return water valve.
Assembly process
Collect the electrolyzer with your own hands according to the following instructions:
- First of all, you need to mark and the subsequent sawing of the stainless steel sheet into identical squares. Sawing can be done with an angle grinder (angle grinder). One of the corners in such squares must be cut at an angle to secure the plates correctly;
- Next, you need to make a hole for the bolt on the side of the plate opposite from the corner saw cut;
- The connection of the plates should be done in turn: one plate on "+", the next on "-" and so on;
- Between the differently charged plates there should be an insulator, which acts as a tube from the spirit level.It should be cut into rings, which should be cut lengthwise to obtain strips of 1 mm thickness. This distance between the plates is sufficient for good gas evolution during electrolysis;
- The plates are fastened together using washers as follows: a washer sits on the bolt, then a plate, then three washers, after a plate, and so on. Plates, favorably charged, are placed in a mirror image of negatively charged sheets. This makes it possible to prevent the sawed edges from touching the electrodes;


Plates of the electrolysis plant assembled together
- When assembling the plates, you should simultaneously isolate them and tighten the nuts;
- Also, each plate must be ringed in order to be sure that there is no short circuit;
- Further, the entire assembly must be placed in a plastic box;
- After that, it is worth highlighting the places where the bolts touch the walls of the container, where you drill two holes. If the bolts do not fit into the container, then they need to be cut with a hacksaw;
- Then the bolts are tightened with nuts and washers for the tightness of the structure;


Plates placed in a plastic container
- After the steps taken, you will need to make holes in the container lid and insert the fittings into them. Impermeability in this case can be ensured by sealing the joints with silicone-based sealants;
- A safety valve and filter in the structure is located at the outlet of the gas and serves as a means of controlling excessive accumulation of gas, which can lead to poor results;
- The electrolysis unit is assembled.
The last stage is a test, which is performed in a similar way:
- filling the container with water up to the mark of the bolts for fasteners;
- connecting power to the device;
- connection to the fitting of the tube, the opposite end of which is lowered into the water.
If a weak current is applied to the installation, then the release of gas through the tube will be almost imperceptible, but it will be possible to watch it from inside the electrolyzer. By increasing the alternating current, adding an alkaline catalyst to the water, it is possible to significantly increase the yield of the gaseous substance.
The made electrolyzer, as a rule, is an important part of many devices, for example, a hydrogen burner.


the appearance of a hydrogen burner, the basis of which is considered to be a self-made electrolyzer
Knowing the types, key characteristics, device and working principle of ionic installations, you can perform the correct assembly of a self-made structure, which is an excellent assistant in a variety of everyday situations: from welding and saving fuel consumption of motor vehicles to the functioning of heating systems.
Device and principle of operation
Cathode and anode
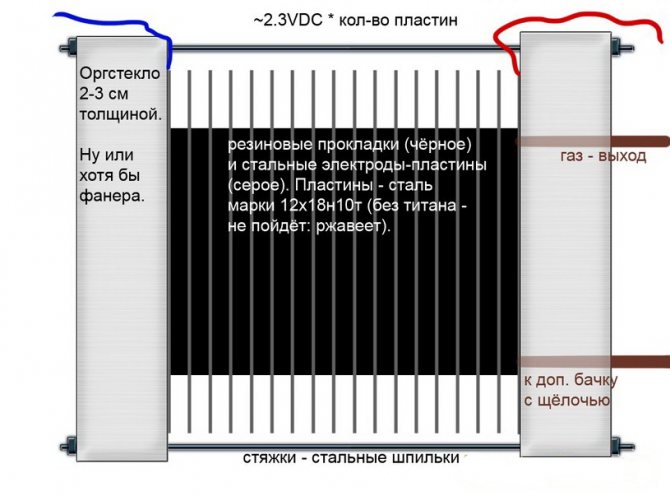
The simplest electrolysis plant consists of several "cells", each of which includes:
- 2 plate electrodes - cathode (negative) and anode (positive);
- A rubber gasket around the perimeter of two adjacent dissimilar electrodes.
The outer cells are equipped with special pipes through which the evolved gases are discharged.
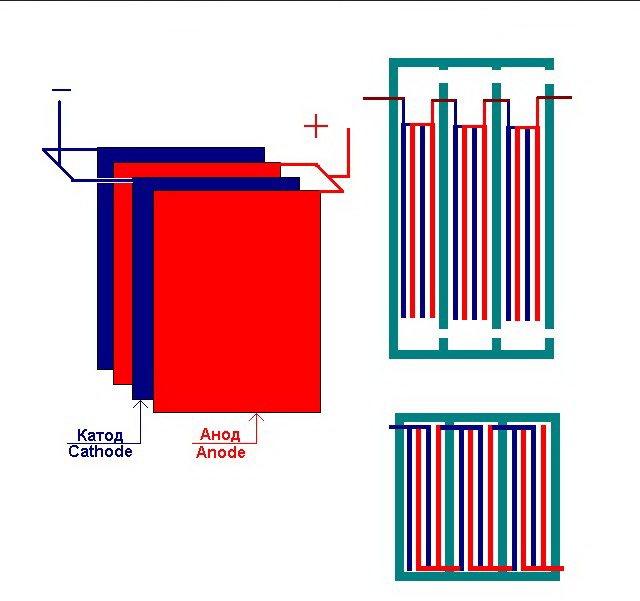

Several interconnected "cells" of the electrolysis plant
The electrolyzer can contain from 1 to 30-40 or more such "cells", the plates of the same name are connected in series.
Important! When using power supplies with alternating current, rectifiers are additionally used, the simplest of which is a diode bridge.
Such an installation works as follows:
- Distilled water with alkali dissolved in it or ordinary baking soda is poured into the space between the electrodes;
- A voltage of 1.8-2.0 V is supplied from the power supply to the electrodes of all cells of the installation;
- As a result of the electrolysis process, anions (positively charged ions) of a substance dissolved in water are attracted to the negatively charged cathode, as a result of which a thin sodium film is formed on it;
- On a positively charged anode, the destruction of water molecules occurs, with each formed 2 hydrogen atoms and 1 oxygen atom;
- The released detonating gas through the branch pipes enters the container intended for it.
The intensity of the electrolysis process depends on the voltage and current strength - at low values of these characteristics, the process will not proceed. If the power source supplies a current with too high values of the current-voltage characteristic, the solution poured into the electrolyzer will be very hot and boil away.
Debugging and testing of the device
Then it is necessary to determine where the bolts touch the walls of the box and, in those places, drill two holes. If for no apparent reason it turns out that the bolts do not fit into the container, then they should cut and tighten for tightness with nuts... Now you need to drill out the cover and insert the threaded connectors there from both sides. To ensure impermeability, the joint should be sealed with a silicone based sealant.
After assembling your own electrolyzer with your own hands, you should test it. To do this, connect the device to a power source, fill it with water to the bolts, put on the lid by connecting a tube to the fitting and lowering the opposite end of the tube into the water. If the current is weak, then the current will be visible from inside the electrolyzer.
Gradually increase the current in your homemade appliance. Distilled water does not conduct electricity well because it contains no salts or impurities. To prepare the electrolyte, it is necessary to add alkali to the water. To do this, you need to take sodium hydroxide (contained in means for cleaning pipes such as "Mole"). A safety valve is needed to prevent a decent amount of gas from accumulating.
- It is better to use distilled water and soda as a catalyst.
- You should mix some of the baking soda with forty parts of water. The walls on the sides are best made of acrylic glass.
- The electrodes are best made of stainless steel. It makes sense to use gold for plates.
- Use translucent PVC for backing. They can be 200 by 160 millimeters in size.
- You can use your own electrolyzer, made by yourself, to cook food, for the complete combustion of gasoline in cars and in most cases.
Dry electrolyzers are mainly used for machines. The generator increases the power of the combustion engine. Hydrogen ignites much faster than liquid fuel, increasing the force of the piston. In addition to Mole, you can take Mister Muscle, caustic soda, baking soda.
The generator does not work on drinking water. It is better to connect electricity like this: the first and the last plate - minus, and on the plate in the middle - plus. The larger the area of the plates and the stronger the current, the more gas is released.
Types of electrolyzers
DIY spot welding for batteries
Depending on the design and principle of operation, there are 5 types of electrolysis plants.
Dry
These electrolyzers consist of plate electrodes separated by sealed rubber gaskets. Often the "cells" of the installation are additionally placed in a sealed enclosure.
Hydrogen and oxygen generated as a result of electrolysis are removed through special branch pipes located at the end of the body or the extreme plates of the installation.
Flowing
Electrolysis installations of this type have the following device:
- An electrolysis bath (body) with two nozzles, through one of which electrolyte is fed into it, through the second the detonating gas formed as a result of electrolysis is discharged;
- Plate electrodes separated by spacers;
- A tank with electrolyte located above the housing with electrodes and connected by hoses to the branch pipes of the electrolysis bath of the installation and having a branch pipe with a gas valve in the upper part.
During the operation of such a device, the evolved gas through the branch pipe and the hose enters the tank with the electrolyte and, creating a certain pressure in it, leaves the installation through the valve on the branch pipe.
Membrane
The electrolysis cells of such installations consist of two electrodes separated by a thin membrane that allows electrolysis products to pass through and separates the electrodes from each other.
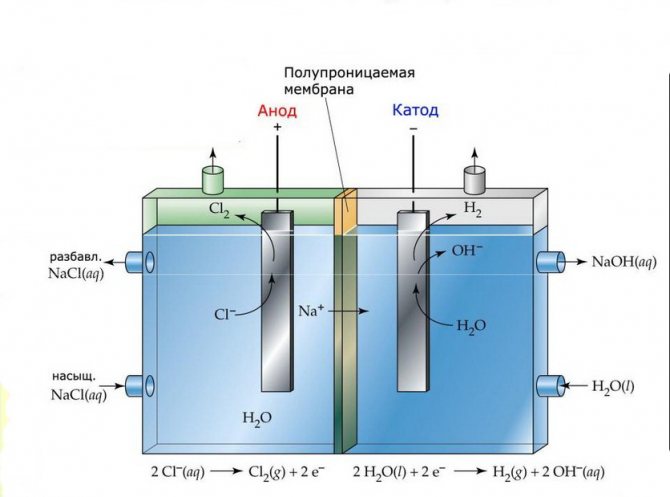

Membrane electrolysis plant
Diaphragm
Electrolysis installations of this type consist of a "U" -shaped flask with two electrodes inserted into it and 2-3 impermeable diaphragms. Similar electrolyzers are used for the separate production of pure hydrogen and oxygen.
Alkaline
Unlike other models of electrolyzers, in these, an alkali solution is used as an electrolyte - caustic soda (sodium hydroxide), which is not only an additional source of hydrogen and oxygen, but also a catalyst for electrolysis.

Alkaline electrolyser circuit
Such installations, in contrast to analogs of other types, allow the use of cheaper electrodes made of ordinary iron.
Do the electrolyser with your own hands
Surely, you are familiar with the electrolysis process from the elementary school curriculum. This is when 2 polar electrodes are placed in water under current in order to obtain metals or non-metals in their pure form. An electrolyzer is needed to decompose water molecules into oxygen and hydrogen. The electrolyser, as part of scientific mechanisms, divides molecules into ions.
There are two types of this device:
- Dry electrolyzer (this is a completely closed cell);
- Wet electrolyzer (these are two metal plates placed in a container of water).
This device is simple in terms of the device, which makes it possible use even at home... Electrolyzers divide the electrolysis charges of the atoms of the molecules into charged atoms.
In our case, it divides water into positive hydrogen and negative oxygen. To do this, a large amount of energy is required, and to make less of the required amount of energy, a catalyst is used.
Electrolyzer for hydrogen production
Grounding calculation
In order to assemble the simplest electrolyzer with your own hands, you can use the drawing shown in the figure.
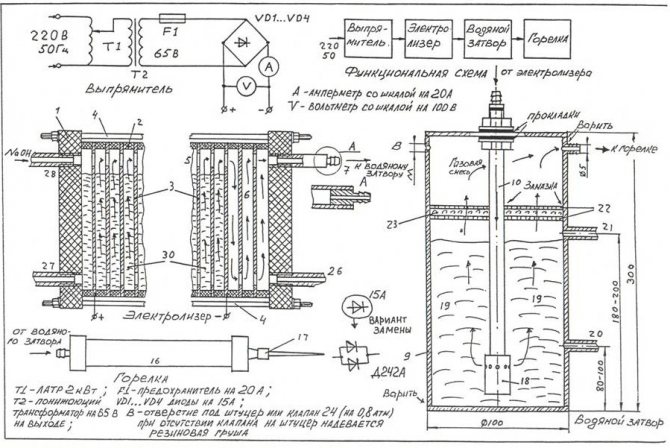

Drawing of the simplest homemade electrolyzer
Note! An electrolyzer is a rather dangerous installation, which, during operation, oxyhydrogen gas, accumulating in large quantities, can cause a serious explosion. The unit should not be placed near sources of open fire, heating devices.
DIY electrolyser for a car
To improve the combustion of fuel in a car engine, an electrolyzer is often assembled, consisting of a case from an old battery with stainless steel plates placed inside, two nozzles, one of which is connected to a tank filled with electrolyte, the second is connected to an air supplying hose to the engine (more precisely, with a corrugated from the air intake to the air filter).
Such a homemade flowing electrolyzer is powered from the car's battery using a relay and a 10 A fuse.
We create a device with our own hands
The device for this process can be done by hand.
For this you will need:
- Stainless steel sheet;
- Bolts M6 x 150;
- Washers;
- Nuts;
- Transparent tube;
- Connecting elements with thread on both sides;
- One and a half liters plastic container;
- Water filter;
- Check valve for water.
An excellent option for stainless steel is AISI 316L of a foreign manufacturer or 03X16H15M3 of a manufacturer from our country. There is absolutely no need to purchase stainless steel, you can take the old one. 50 to 50 centimeters is enough for you.
"Why take stainless steel itself?" - you ask. Since the most common metal will corrode. Stainless steel tolerates alkalis better. Should outline the sheet in such a way as to divide it into 16 similar squares... You can cut it with an angle grinder. In each square, cut one of the corners.
On the other side and opposite corner, from the sawn-off corner, drill a hole for a bolt that will help hold the plates together. The electrolyzer does not stop working like this:t plate electricity flows to the plate - and water decomposes into oxygen and hydrogen. Thanks to this, we need a good and negative plate.
Plates must be connected alternately: plus-minus-plus-minus, with a similar method, there will be a strong current. To insulate the plates one from one, a tube is used. A ring is cut from the level. By cutting it, we get a strip millimeter thick. This distance is more correct for making gas.
The plates are interconnected with washers: we put a washer on the bolt, then a plate and three washers, then a plate again, and so on. On the plus and minus, eight plates must be planted. If everything is done correctly, then the cuts of the plates will not touch the electrodes.
Then you need to tighten the nuts and isolate the plates. Then we place the structure in a plastic container.
Electrolyzer manufacturers overview
The main producers of electrolysers are both domestic enterprises (RUSAL, NPF RutTech, JSC Uralkhimmash), as well as their foreign competitors - Teledyne Energy Systems, Inc (USA), Hydrogenics Corp. (Belgium).
Thus, the electrolyzer is a fairly simple and feature-rich installation used to produce oxyhydrogen gas, which in the future is planned to be used as fuel for internal combustion engines and heating boilers.
Do-it-yourself home electrolysis
When I was small, I always wanted to do something myself, with my own hands. But the parents (and other close people) in most cases did not allow this. And I did not see then (and until now do not see) anything wrong when little children want to learn ??
Of course, I did not write this article in order to recall childhood experiences in the desire to start self-education. Just by accident, when I was surfing the Internet, I came across a question of this kind. Some little bomber boy asked questions about how to do electrolysis at home. True, I did not answer him, because this boy wanted to electrolyze the painfully suspicious mixture ?? I decided that I would not say further out of sin, let me look in books myself. But not so long ago, again wandering through the forums, I saw a similar question from a teacher at a chemistry school. Judging by the description, his school is so poor that it cannot (does not want to) buy an electrolyzer for 300 rubles. The teacher (what a problem!) Could not find a way out of the resulting situation. So I helped him. For those who are curious about this kind of homemade products, I post this article on the site.
Actually, the production process and the use of our self-pallet is very primitive. But I will tell you about safety first, and about manufacturing - in the second. And the point is that we are talking about a demonstration electrolyzer, and not about an industrial installation. Thanks to this, for safety it will be good to power it not from the network, but from AA batteries or from a battery. Naturally, the higher the voltage, the faster the electrolysis process will go. However, for visual observation of gas bubbles, it is quite enough 6 V, but 220 is already excessive. with such a voltage, water, for example, will boil the fastest, and this is not very safe ... Well, I think you figured out the tension?
Now let's talk about where and under what conditions we will experiment. The very first thing, it should be either free space or a well-ventilated room. Although I did everything in an apartment with closed windows and nothing like? Second, the experiment is best done on a good table. The word "good" means that the table must be stable, or better, heavy, rigid and attached to the floor surface. In this case, the table covering must be resistant to aggressive substances. By the way, tile from a tile is perfect for this (although not every, unfortunately). A table like this will come in handy not only for this experience. However, I did everything on an ordinary stool ?? Third, during the experiment, you do not need to move the power source (in my case, batteries). Due to this, for reliability, it is best to immediately lay them on the table and fix them so that they do not budge. Believe me, this is more convenient than holding them regularly with your hands. I simply tied my own batteries with electrical tape to the first hard object I saw. Fourthly, the dishes in which we will experiment, let them be small. A simple glass fits or a shot glass. By the way, this is the most optimal way to use glasses at home, as opposed to pouring alcohol into them with further use ...
Well, now let's move on specifically to the device. It is provided in the figure, but for now I will briefly explain what and what.
We need to take a simple pencil and remove the tree from it with an ordinary knife and get a whole lead out of the pencil. You can, however, take a lead from a mechanical pencil. But there are two difficulties at once. The first is the usual one. The lead from a mechanical pencil is too thin, for us this is simply not suitable for a visual experiment. The second difficulty is some incomprehensible composition of the current slates. It feels like they are not made from graphite, but from something else. In general, my experience with such a "lead" was not successful at all, even at a voltage of 24 V. Thanks to this, I needed to pick out a good woody simple pencil. The resulting graphite rod will serve as an electrode for us. As you can imagine, we need two electrodes. Thanks to this, we go to pick the second pencil, or simply break the existing rod in two. I actually did this.
With any wire that comes to hand, we wrap the first lead-electrode (with one end of the wire), and we connect this wire to the minus of the power source (with the other end). Then we take the second lead and do the same with it. For this, based on this, we need a second wire. But in this case, we connect this wire to the plus of the power supply. If you have problems attaching the fragile graphite rod to the wire, you can use the tools at hand, such as tape or duct tape. If it did not work out to wrap the tip of the graphite with the wire itself, and the tape or insulating tape did not provide a tight contact, then try to glue the lead with conductive glue. If you don't have this, then at least tie the lead to the wire with a thread. No need to be afraid, the thread will not burn out from such tension ??
For those who do not know anything about batteries and the simple rules for connecting them, I will explain a little. The finger-type battery produces a voltage of 1.5 V. In the picture I have two similar batteries. Moreover, they are connected gradually - one after the other, not in parallel. With a similar (serial) connection, the final voltage will be summed up from the voltage of each battery, that is, for me it is 1.5 + 1.5 = 3.0 V. This is less than the previously stated 6 volts. But I was too lazy to go buy a few more batteries. Principle you and so must be clear ??
Let's start the experiment. For example, we will restrict ourselves to the electrolysis of water.First, it is very accessible (I hope that the reader of this article does not live in the Sahara), and second, it is harmless. Moreover, I will show how with the same device (electrolyzer) with the same substance (water) to perform two various experience. I think that you have enough imagination to come up with a bunch of similar experiments with other substances ?? In general, tap water is suitable for us. But I recommend that you add a little more of it and salt it. A little bit - this means a small pinch, not a whole dessert spoon. This is important! Stir the salt well to dissolve. So water, being a dielectric in a pure state, will perfectly conduct electricity. at the beginning of the experiment, wipe the table from potential moisture, and then put the power source and a glass of water on it.
We lower both electrodes, present under voltage, into the water. At the same time, make sure that only graphite is immersed in the water, and the wire itself should not touch the water. The beginning of the experiment may be delayed. Time depends on many indicators: on the composition of the water, the quality of the wires, the quality of the graphite and, of course, the voltage of the power source. The beginning of my reaction was delayed for a couple of seconds. Oxygen begins to evolve on the electrode that was connected to the plus of the batteries. Hydrogen will be released on the electrode connected to the minus. It should be noted that there are more hydrogen bubbles. Very small bubbles stick around the part of the graphite that is submerged in the water. Then some of the bubbles start to float.
Electrode at the beginning of the experiment. There are no gas bubbles yet. Hydrogen bubbles formed on the electrode connected to the negative pole of the batteries
What other experiments can there be? If you have already played enough with hydrogen and oxygen, we proceed to another experiment. It is more interesting, especially for home researchers. It is interesting in that it is possible not only to see it, but also to smell it. In the past experience, we received oxygen and hydrogen, which, in my opinion, are not very spectacular. And in another experiment, we get two substances (useful in everyday life, by the way). at the beginning of the experiment, stop the previous experiment and dry the electrodes. Now take table salt (which you use in most cases in the kitchen room) and dissolve it in the water mass. In this case, not a small amount. Actually, a decent amount of salt is the only thing that makes the second experience different from the first. After dissolving the salt, you can immediately repeat the experiment. Now a different reaction is taking place. On a good electrode, it is not oxygen that is released now, but chlorine. And on the negative, hydrogen is also released. As for the glass in which the salt solution is located, sodium hydroxide remains in it after prolonged electrolysis. This is the familiar caustic soda, alkali.
Chlorine, you will be able to smell it. But for the best effect, I recommend taking a voltage of at least 12 V. Otherwise, you may not feel the aroma. The presence of alkali (after a very long electrolysis) in the glass can be checked in several ways. The simplest and most violent is to put your hand in the glass. An ethnic omen says that if a burning sensation begins, there is alkali in the glass. A smarter and more distinct way is the litmus test. If your school is so poor that it is not even able to acquire a litmus, the indicators at hand will help you. One of these, as they say, can serve as a drop of beet juice ?? But it is quite possible to just drip a little fat into the solution. As far as I know, saponification should take place.
For the very curious, I will describe what actually happened during the experiments. In the first experiment, under the influence of an electric current, a similar reaction took place: 2 H2O >>> 2 H2 + O2 Both gases naturally float from the water to the surface. By the way, floating gases can be trapped. Will you be able to do it yourself?
In another experiment, the reaction was completely different.It was also initiated by an electric current, but now not only water, but also salt acted as reagents: 4H2O + 4NaCl >>> 4NaOH + 2H2 + 2Cl2 Keep in mind that the reaction must take place in an excess of water. To find out what amount of salt is considered the largest, you can count it from the above reaction. You can also think about how to improve the device or what other experiments can be done. Indeed, it is possible that sodium hypochlorite can be obtained by electrolysis. In laboratory conditions, in most cases, it is obtained by passing gaseous chlorine through a sodium hydroxide solution.