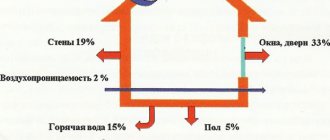
Most of all, in the frosty winter months, all people are waiting for the New Year, and least of all - receipts for heating. They are especially disliked by residents of apartment buildings, who themselves do not have the ability to control the amount of incoming heat, and often the bills for it turn out to be simply fantastic. In most cases, in such documents, Gcal is used as a unit of measurement, which stands for "gigacalorie". Let's find out what it is, how to calculate gigacalories and convert to other units.
What is called a calorie
Supporters of a healthy diet or those who strenuously monitor their weight are familiar with such a concept as a calorie. This word means the amount of energy received as a result of the processing of food eaten by the body, which must be used, otherwise a person will begin to recover.

Paradoxically, the same value is used to measure the amount of thermal energy used to heat rooms.
In physics, it is generally accepted that one calorie is the amount of energy required to heat one gram of H2O per 1 ° C at standard atmospheric pressure (101,325 Pa).


As an abbreviation, this value is referred to as "feces", or in English cal.
In the metric system, the joule is considered the equivalent of a calorie. So, 1 cal = 4.2 J.
The importance of calories for human life
Besides developing various weight loss diets, this unit is used to measure energy, work and warmth. In this regard, such a concept as "calorie content" is widespread - that is, the heat of the combustible fuel.
In most developed countries, when calculating heating, people no longer pay for the amount of consumed cubic meters of gas (if it is gas), but precisely for its calorie content. In other words, the consumer pays for the quality of the fuel used: the higher it is, the less gas will have to be consumed for heating. This practice reduces the possibility of diluting the substance used with other, cheaper and less caloric compounds.


Power units
Power is measured in joules per second, or watts. Along with watts, horsepower is also used. Before the invention of the steam engine, the power of engines was not measured, and, accordingly, there were no generally accepted units of power. When the steam engine began to be used in mines, engineer and inventor James Watt began to improve it. In order to prove that his improvements made the steam engine more efficient, he compared its power to the performance of horses, since horses have been used by people for many years, and many could easily imagine how much work a horse could do in a given amount of time. In addition, steam engines were not used in all mines. On those where they were used, Watt compared the power of the old and new models of the steam engine with the power of one horse, that is, with one horsepower. Watt determined this value experimentally by observing the work of draft horses at a mill. According to his measurements, one horsepower is 746 watts. Now it is believed that this figure is exaggerated, and the horse cannot work in this mode for a long time, but they did not change the unit. Power can be used as an indicator of productivity, since as power increases, the amount of work performed per unit of time increases. Many people realized that it was convenient to have a standardized unit of power, so horsepower became very popular. It began to be used to measure the power of other devices, especially transport.Although watts are used almost as long as horsepower, the automotive industry is more likely to use horsepower, and many buyers have a better understanding of when these units are used to indicate the power of an automobile engine.
What is a gigacalorie and how many calories are in it?
As it is clear from the definition, the size of 1 calorie is small. For this reason, it is not used for calculating large quantities, especially in power engineering. Instead, a concept such as a gigacalorie is used. This value is equal to 109 calories, and it is written in the form of abbreviation "Gcal". It turns out that there are one billion calories in one gigacalorie.
In addition to this value, a somewhat smaller one is sometimes used - Kcal (kilocalorie). It holds 1000 calories. Thus, we can assume that one gigacalorie is a million kilocalories.
It should be borne in mind that sometimes a kilocalorie is recorded simply as "feces". Because of this, confusion arises, and in some sources it is indicated that 1 Gcal - 1,000,000 calories, although in reality it is about 1,000,000 Kcal.
Gigacalorie and gigacalorie / hour: what's the difference
In addition to the fictitious value under consideration, such abbreviations as "Gcal / hour" are sometimes found in receipts. What does it mean and how does it differ from the usual gigacalorie?
This unit of measure shows how much energy was used in one hour.


While just a gigacalorie is a measure of the consumed heat for an indefinite period of time. It depends only on the consumer what time frames will be indicated in this category.
Reduction of Gcal / m3 is much less common. It means how many gigacalories you need to use to heat one cubic meter of a substance.
This is the ratio of Cal and Gcal to each other.
1 Cal 1 hectoCal = 100 Cal 1 kiloCal (kcal) = 1000 Cal 1 megaCal (Mcal) = 1000 kcal = 1,000,000 Cal 1 gigaCal (Gcal) = 1,000 Mcal = 1,000,000 kcal = 1,000,000,000 Cal
When, speaking or writing in receipts, Gcal
- we are talking about how much heat was released to you or released for the entire period - it can be a day, month, year, heating season, etc.
When they say
or write
Gcal / hour
- it means, . If the calculation is carried out for a month, then we multiply these ill-fated Gcal by the number of hours per day (24 if there were no interruptions in heat supply) and days per month (for example, 30), but also when we received heat in fact.
And now how to calculate this very gigacalorie or hecocaloria (Gcal) released to you personally.
To do this, we need to know:
- temperature at the supply (supply pipeline of the heating network) - average value per hour; - the temperature on the return (the return pipe of the heating network) is also an hourly average. - the flow rate of the coolant in the heating system for the same period of time.
We consider the temperature difference between what came to our house and what came back from us to the heating network.
For example: 70 degrees came, we returned 50 degrees, we have 20 degrees left. And we also need to know the water consumption in the heating system. If you have a heat meter, we are perfectly looking for the value in t / hour
... By the way, using a good heat meter, you can immediately
find Gcal / hour
- or, as they sometimes say, instantaneous consumption, then there is no need to count, just multiply it by hours and days and get heat in Gcal for the range you need.
True, this will also be approximately, as if the heat meter counts for every hour itself and organizes it into its archive, where you can always look at them. Average keep hourly archives for 45 days
, and menstruation up to three years. Readings in Gcal can always be found and checked by the management company or.
Well, what if there is no heat meter. You have a contract, there are always these ill-fated Gcal. We will calculate the consumption in t / h using them. For example, the contract says that the permitted maximum heat consumption is 0.15 Gcal / hour.It can be written differently, but Gcal / hour will always be. 0.15 is multiplied by 1000 and divided by the temperature difference from the same contract. You will have a temperature graph indicated - for example, 95/70 or 115/70 or 130/70 with a cut of 115, etc.
0.15 x 1000 / (95-70) = 6 t / h, these 6 tons per hour are what we need, this is our planned pumping (coolant consumption) to which we must strive so as not to have overheating and underfilling (unless of course in the contract, you correctly indicated the value of Gcal / hour)
And, finally, we count the heat received earlier - 20 degrees (the temperature difference between what came to our house and what came back to the heating network) multiplied by the planned pumping (6 t / h) we get 20 x 6/1000 = 0.12 Gcal / hour.
This amount of heat in Gcal released to the whole house, the management company will personally calculate it for you, usually this is done by the ratio of the total area of the apartment to the heated area of the whole house, I will write more about this in another article.
The method described by us is, of course, rough, but for every hour this method is possible, just keep in mind that some heat meters averaged the flow rates for different time intervals from a few seconds to 10 minutes. If the water consumption changes, for example, who disassembles the water, or you have a weather-dependent automation, the readings in Gcal may differ slightly from those obtained by you. But this is on the conscience of the developers of heat meters.
And one more small note, the value of the consumed heat energy (amount of heat) on your heat meter
(heat meter, calculator of the amount of heat) can be displayed in various units of measurement - Gcal, GJ, MWh, kWh. I give the ratio of units of Gcal, J and kW for you in the table: Better, more accurate and simpler if you use a calculator to convert energy units from Gcal to J or kW.
In receipts for heating, the measurement can be used:
- Gcal;
- Gcal / hour.
In the first case, we mean the supplied heat for a certain period (this can be a month, a year or a day). Gcal / hour is a characteristic of the power of a device or process (such a unit of measurement can report the performance of a heater or the rate of heat loss of a building in winter). In receipts, heat is meant, which was released in 1 hour. Then, to recalculate for a day, you need to multiply the number by 24, and for a month by another 30/31.
1 Gcal / hour = 40 m 3 of water, which was heated to 25 ° C in 1 hour.
Also, a gigacalorie can be tied to the volume of fuel (solid or liquid) Gcal / m3. And it shows how much heat can be obtained from a cubic meter of this fuel.
Gigacalorie formula
Having considered the definition of the studied value, it is worth finally learning how to calculate how many gigacalories are used to heat a room during the heating season.
For especially lazy people on the Internet, there are a lot of online resources where specially programmed calculators are presented. It is enough to enter your numerical data into them - and they themselves will calculate the amount of consumed gigacalories.


However, it would be nice to be able to do it yourself. There are several formula options for this. The simplest and most understandable among them is the following:
Heat energy (Gcal / hour) = (М1 х (Т1-Тхв)) - (М2 х (Т2-Тхв)) / 1000, where:
- M1 is the mass of the heat transfer substance that is supplied through the pipeline. Measured in tons.
- M2 is the mass of the heat transfer substance returning through the pipeline.
- T1 is the temperature of the coolant in the supply pipeline, measured in Celsius.
- T2 is the temperature of the coolant returning back.
- Тхв - temperature of the cold source (water). Usually equal to five degrees Celsius, since this is the minimum temperature of the water in the pipeline.
General principles for performing gcal calculations
The calculation of kW for heating implies the performance of special calculations, the order of which is regulated by special regulations. Responsibility for them lies with utilities, which are able to help with this work and give an answer on how to calculate gcal for heating and decoding gcal.
Of course, such a problem will be completely eliminated if there is a hot water meter in the living room, since it is in this device that there are already pre-set readings that reflect the received heat. Multiplying these results by the established tariff, it is fashionable to obtain the final parameter of the consumed heat.
Why do housing and communal services overestimate the amount of energy spent when calculating for heating?
Carrying out your own calculations, you should pay attention to the fact that housing and communal services slightly overestimate the standards for thermal energy consumption. The opinion that they are trying to earn extra money on this is wrong. After all, the cost of 1 Gcal already includes services, salaries, taxes, and additional profit. Such a "surcharge" is due to the fact that when hot liquid is transported through a pipeline in the cold season, it tends to cool down, that is, inevitable heat loss occurs.


In numbers, it looks like this. According to the regulations, the temperature of the water in the pipes for heating must be at least +55 ° C. And if we take into account that the minimum t of water in power systems is +5 ° C, then it must be heated by 50 degrees. It turns out that 0.05 Gcal is used for each cubic meter. However, to compensate for heat loss, this coefficient is overestimated to 0.059 Gcal.
Conversion of Gcal to kW / hour
Thermal energy can be measured in various units, but in the official documentation from housing and communal services it is calculated in Gcal. Therefore, it is worth knowing how to convert other units to gigacalories.
The easiest way to do this is when the ratio of these quantities is known. For example, consider the watts (W), which measures the power output of most boilers or heaters.
Before considering the conversion of Gcal to this value, it is worth remembering that, like a calorie, a watt is small. Therefore, more often use kW (1 kilowatt, equal to 1000 watts) or mW (1 megawatt equals 1000,000 watts).
In addition, it is important to remember that power is measured in W (kW, mW), but kW / h (kilowatt-hours) is used to calculate the amount of electricity consumed / produced. In this regard, it is not the conversion of gigacalories to kilowatts that is considered, but the conversion of Gcal to kW / h.


How can this be done? In order not to bother with the formulas, it is worth remembering the "magic" number 1163. That is how many kilowatts of energy you need to spend in an hour to get one gigacalorie. In practice, when converting from one unit of measurement to another, it is simply necessary to multiply the amount of Gcal by 1163.
For example, let's convert 0.05 Gcal in kWh / hour required to heat one cubic meter of water by 50 ° C. It turns out: 0.05 x 1163 = 58.15 kW / hour. These calculations will especially help those who are thinking about switching from gas heating to a more environmentally friendly and economical electric one.
If we are talking about huge volumes, it is possible to translate not into kilowatts, but into megawatts. In this case, you need to multiply not by 1163, but by 1.163, since 1 mW = 1000 kW. Or simply divide the result in kilowatts by a thousand.
Energy in physics
Kinetic and potential energy
Kinetic energy of a body mass m
moving at speed
v
equal to the work done by the force to give the body speed
v
... Work is defined here as a measure of the action of a force that moves a body a distance
s
... In other words, it is the energy of a moving body. If the body is at rest, then the energy of such a body is called potential energy. This is the energy required to keep the body in this state.
For example, when a tennis ball hits a racket in flight, it stops momentarily. This is because the forces of repulsion and gravity cause the ball to freeze in the air. At this moment, the ball has potential, but no kinetic energy. When the ball bounces off the racket and flies away, on the contrary, it has kinetic energy. A moving body has both potential and kinetic energy, and one type of energy is converted into another. If, for example, toss up a stone, it will begin to slow down during flight. As this slows down, kinetic energy is converted into potential energy. This transformation takes place until the supply of kinetic energy is exhausted. At this moment, the stone will stop and the potential energy will reach its maximum value. After that, it will begin to fall downward with acceleration, and the transformation of energy will occur in the reverse order. Kinetic energy will peak when the rock hits the ground.
The law of conservation of energy states that the total energy in a closed system is conserved. The energy of the stone in the previous example changes from one form to another, and therefore, despite the fact that the amount of potential and kinetic energy changes during flight and fall, the total sum of these two energies remains constant.